Prepared by Dr. Hussein Rasool Abid

CO2 gas is a main gas caused Global warming therefore reducing concentration of this gas has been worldly required. CO2 emission is required to be dropped to zero-emission. There are two solutions to decarbonate the CO2 discharged from industrial sources; the first is to capture this CO2 using novel hybrid materials with high surface area and a good functionality. Physisorption method (Figure 1) is the most reliable one to capture huge amount of CO2 and then the adsorbed CO2 is successfully stored in a suitable giant storage (Underground).

Figure 1. Fixed-bed column adsorption.
The second method is made based on the electrochemical theory; therefore, electrolysis process is successfully established using H2O to produce pure H2 and O2. Then, this method can convert CO2 to another beneficial syn gas (CO+H2) by co- electrocatalysis process at high temperature using H2O and CO2 in Solid oxide electrolysis cell (SOEC). Noteworthy to mention, it is possible to use both chemisorption and co-electrolysis processes to capture CO2 and convert it to useful fuel as shown in Figure 2. CO2 and H2O are passed through SOEC under heating at temperature of 600-degree C. On the cathode, the catalysis process will be occurred to promote the splitting of CO2 and water producing hydrogen ions (H+), Oxygen ions (O–) and carbon monoxide (CO) as shown in below reactions. As a result of the chemical reactions on the cathode, hydrogen (H2), CO, and oxygen (O2) will be produced in the final product. Anode, cathode, and electrolyte are the three main components of SOEC as shown in Figure 2.
CO2+ 2e-2 ⇌ CO+O-2
O-2 -e-2 ⇌ 1/2O2
H2O-2e-1 ⇌ 2H+ + 1/2O2
2H++2e-1 ⇌ H2
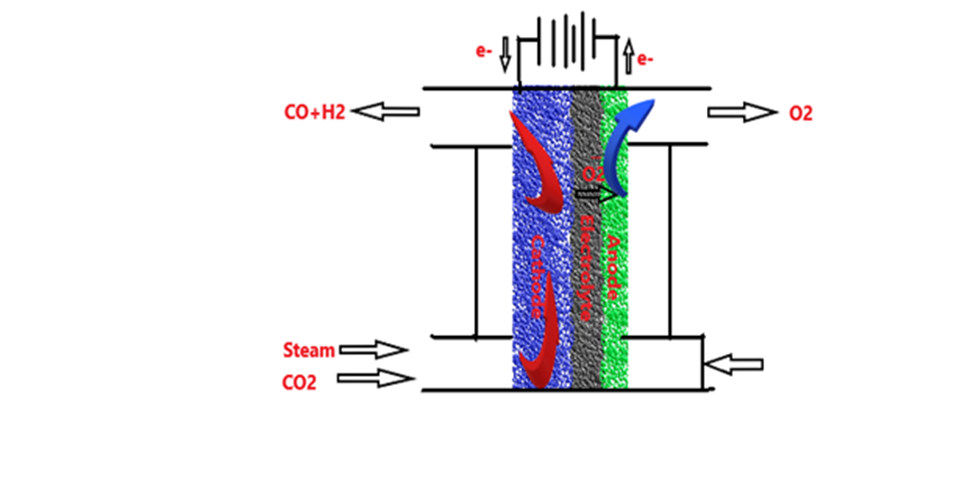
Figure 2. Co-electrolysis Cell of CO2 to H2 and CO




























































